Legal advice
Navigating the complex legal framework surrounding medicinal cannabis in Spain, France, and Portugal can be challenging. This comprehensive guide, brought to you by Agropharm, explores the regulations, compliance requirements, and best practices for businesses and individuals operating in the cannabis industry. With expert legal support, Agropharm helps you ensure that your operations align with the latest legislation, empowering you to thrive in this rapidly evolving sector. Whether you’re seeking to establish a cannabis business, expand your existing operations, or ensure compliance with medicinal cannabis laws, our insights and guidance will pave the way for success.

Legal advice
Navigating the complex legal framework surrounding medicinal cannabis in Spain, France, and Portugal can be challenging. This comprehensive guide, brought to you by Agropharm, explores the regulations, compliance requirements, and best practices for businesses and individuals operating in the cannabis industry. With expert legal support, Agropharm helps you ensure that your operations align with the latest legislation, empowering you to thrive in this rapidly evolving sector. Whether you’re seeking to establish a cannabis business, expand your existing operations, or ensure compliance with medicinal cannabis laws, our insights and guidance will pave the way for success.

AGRONOMIC ADVICE
LIGHTING, SENSORS AND MONITORING TECHNOLOGY…
LEGAL ADVICE
TO COMPLY WITH REGULATIONS
LEGAL ADVICE
TO COMPLY WITH REGULATIONS.
Why choose Agropharm for legal advice in the Cannabis Industry?
At Agropharm, we combine deep expertise in legal and agronomic consultancy to support businesses in the cannabis industry. Our team understands the intricate regulations governing medicinal cannabis across Spain, France, Portugal, and other european countries, ensuring that you receive guidance tailored to your specific needs.
- Expertise You Can Trust: With years of experience in the cannabis sector, we provide advice that not only ensures compliance but also helps optimize your operations for long-term success.
- Commitment to Quality: Our guidance guarantees that your products meet the highest standards of safety and quality, complying with stringent regulations for human consumption.
- Specialized Legal Consultancy: In partnership with Bezanilla Renedo Abogados, and other leading legal firms, we offer specialized support to navigate the complexities of cannabis laws, including licensing, compliance, and market entry strategies in different countries.
Choosing Agropharm means partnering with a team dedicated to helping your business thrive in the regulated cannabis market.
Key Areas of Legal Advice and Support
These are the Key Areas of Legal Advice and Support:
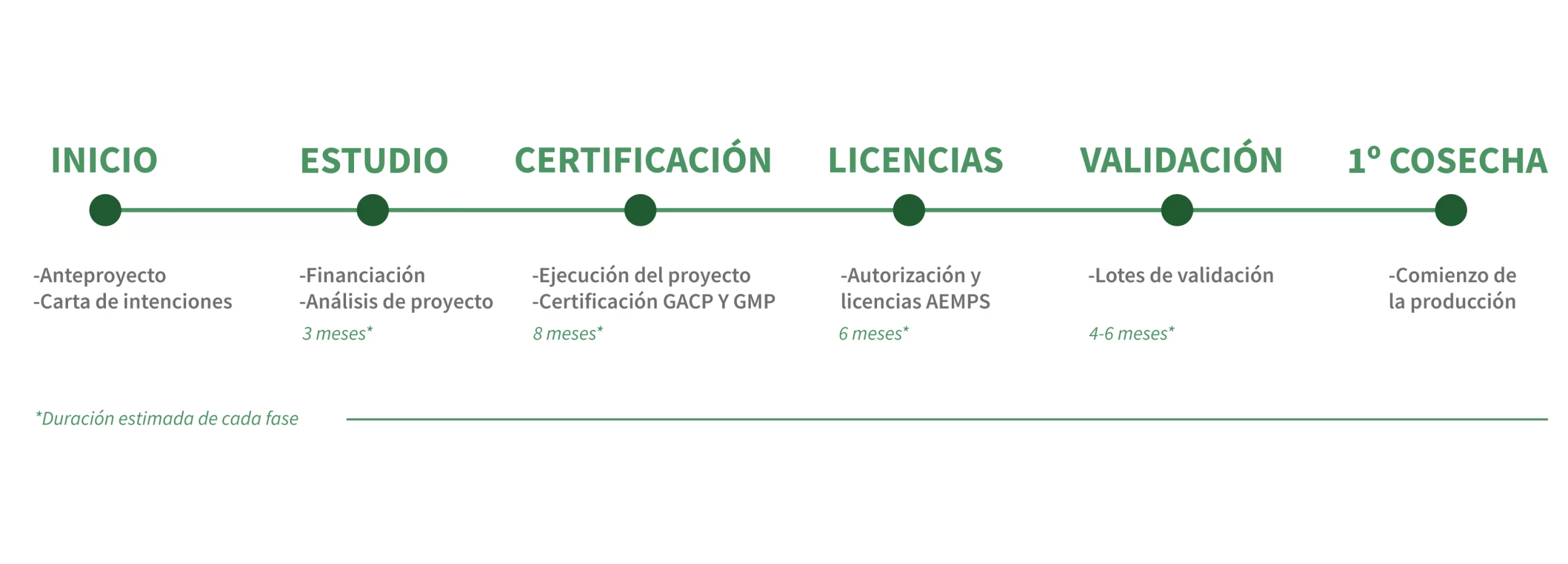
Prior Authorisation for Medicinal Cannabis Cultivation
Before commencing any cultivation project for medicinal cannabis, administrative authorisation is a critical first step. Entities like AEMPS and systems like RUESA in Spain, or entities like INFARMED in Portugal, oversee the approval process, which requires detailed documentation and compliance with national and EU-level regulations.
At Agropharm, we guide you through each stage of this complex procedure, ensuring all requirements—such as site specifications, security measures, and intended use—are thoroughly addressed. Our team’s expertise eliminates uncertainties, streamlining the authorisation process and setting your project on the path to success.
Regulatory Compliance for Medicinal Cannabis
Adhering to stringent medicinal cannabis regulations is essential for maintaining operational integrity and consumer trust. Agropharm ensures compliance with laws in Spain, Portugal, France, and other countries, helping businesses meet rigorous safety and quality benchmarks such as GACP (Good Agricultural and Collection Practices) and GMP (Good Manufacturing Practices) protocols.
From cultivation to processing, we offer comprehensive support to ensure that your products align with these standards, safeguarding both your business and your customers.
Ensuring Harmony in Legal and Operational Processes
A seamless integration between legal frameworks, production protocols, and processing operations is vital for the success of any medicinal cannabis project. Agropharm provides a holistic approach, enabling businesses to align legal requirements with operational efficiency.
Our support extends to obtaining administrative authorisation, optimizing workflows, and implementing compliance measures that harmonize legal and production processes. This ensures that your operations run smoothly, minimizing risks and maximizing productivity
Focused on guaranteeing results
Specialized Legal Consultancy for the Cannabis Industry
We let you here the legal points in which we are specialized in Agropharm:
Comprehensive Legal Consultancy
In collaboration with Bezanilla Renedo Abogados, Agropharm offers a full spectrum of legal services tailored to the cannabis industry. This partnership ensures that every phase of your project—from initial planning to final execution—is supported by expert legal guidance, facilitating smooth navigation through complex regulatory landscapes.
Basic Legal Advice
Establishing the appropriate legal structure is crucial for the success of your cannabis cultivation business. Our team assists in selecting the optimal legal entity, considering factors such as liability, taxation, and compliance. Additionally, we provide guidance on accessing subsidies and incentives available for green economy initiatives, helping you secure financial support to strengthen your operations.
Specific Legal Advice for Cannabis Industry Projects
Navigating the licensing requirements in Spain and Portugal is a complex process. We assist in obtaining necessary authorizations from entities like AEMPS in Spain and INFARMED in Portugal, ensuring all regulatory criteria are met. Our services include implementing safety protocols such as IQ and adhering to quality standards like GAPC and GMP, which are essential for compliance and product excellence. We also specialize in drafting Agricultural Production Agreements (APAs) that are compatible with common law standards, providing a solid legal foundation for your business operations.
Navigating cannabis laws in Spain, France, and Portugal
Operating within the cannabis industry requires a thorough understanding of the legal landscape across different countries. Spain, France, and Portugal each have their own unique regulations governing medicinal cannabis cultivation and production. Agropharm provides expert guidance to help businesses navigate these complex frameworks, ensuring full compliance and seamless operations.
Cannabis laws in Spain
Spain’s medicinal cannabis laws are primarily overseen by AEMPS (Spanish Agency of Medicines and Medical Devices). Businesses looking to cultivate medicinal cannabis must obtain prior authorisation from AEMPS, which requires detailed documentation on cultivation plans, security protocols, and intended use. Agropharm supports clients through this process, ensuring all regulatory requirements are met while streamlining approvals.
Cannabis laws in France
In France, medicinal cannabis cultivation and production are tightly regulated, requiring businesses to meet stringent legal and safety standards. From securing licenses to adhering to quality protocols, compliance is non-negotiable. Agropharm assists clients in navigating these regulations, offering tailored solutions to meet French legal requirements and maintain operational integrity.
Cannabis laws in Portugal
Portugal’s regulatory framework for medicinal cannabis is governed by INFARMED. Securing cultivation and production licenses involves multiple steps, including detailed inspections and adherence to strict compliance protocols. Agropharm simplifies this process by guiding clients through each stage, ensuring that all INFARMED requirements are satisfied and businesses can operate without legal risks.
