Miguel Pinto, CEO and Lab Planner of Pharma Arquitetos, is the moderator of this panel.
How is the design of a medicinal cannabis project organized?
Juan Antonio Márquez, Director of Technical Office at Agropharm Projects:
Agropharm, as a project integrator, first organizes the client’s needs. Then, we seek a solution whose design addresses the client’s challenges, and from that point on, we collaborate with different companies to achieve a final design.
Juan Carlos Jiménez, Commercial Director at Ridder:
Agropharm provides us with information, and we propose, based on our technical solutions, equipment tailored to the project’s needs, building upon the information, equipment, and structure that Agropharm has already designed.
Roberto Español, Technical Director of Life Sciences at Trescal:
Once the design is established and approved in the initial stages, a framework is set up to ensure process repeatability and reproducibility, as well as the safety and effectiveness of the product itself.
We start with the understanding that the project is a dynamic entity that always undergoes changes. It’s essential to understand the risks you’ll encounter in the production stages and how to address them to ensure the project’s safety and robustness.
Raúl Fernández, Project Engineer at Labsom:
We specialize in designing the GMP area of cannabis production plants in collaboration with Agropharm. The most important aspect is having a specific goal and knowing the limiting factors.
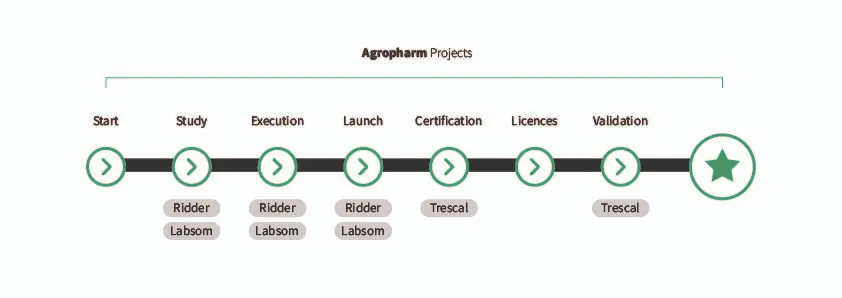
Figure 1. Participation of collaborators in the various project phases.
Miguel Pinto, CEO and Lab Planner of Pharma Arquitetos, posed the second question:
In Portugal, 70-80% of projects fail before they begin.
What are the factors that determine project success?
Juan Antonio Márquez, Director of Technical Office at Agropharm Projects:
There are two fundamental factors: the precise definition of the project and collaboration between companies.
Juan Carlos Jiménez, Commercial Director at Ridder:
For us, success means customer satisfaction. Having the ability to monitor and know exactly what is happening with the cultivation 24 hours a day is a powerful tool, and it greatly exceeds expectations.
Roberto Español, Technical Director of Life Sciences at Trescal:
Being able to predict and control future potential process variations as a result of the advancement and maintenance of the production process.
Raul Fernández, Project Engineer at Labsom:
The keys to success are trust and communication among collaborators, clarity in objectives, and having a “manager” who makes decisions.
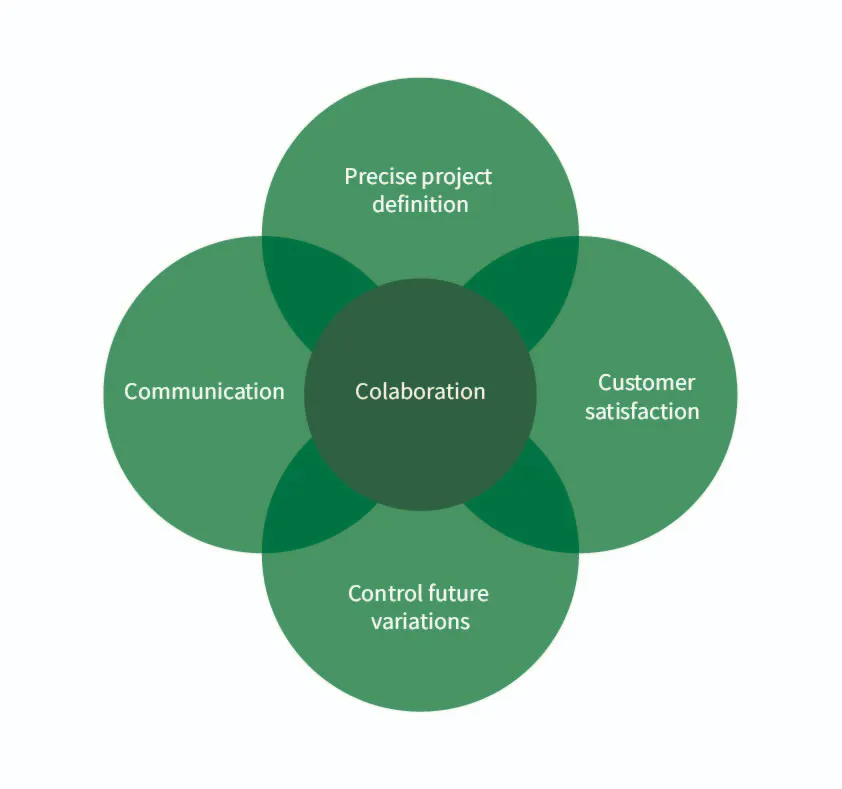
Figure 2. Key factors that lead a project to success.
How long does it take to complete a medicinal cannabis project, from design to commissioning?
Juan Antonio Márquez, Director of Technical Office at Agropharm Projects:
It’s challenging to estimate exact times. Some clients have a clear vision, and we can kickstart the project in 2 or 3 months. As for construction, various factors like weather conditions, material stock, etc., come into play. However, for a standard project, it would typically take 10 to 12 months, depending on the project’s scope.
Juan Carlos Jiménez, Commercial Director at Ridder:
The project itself can be divided into several phases:
Preliminary phase: encompasses the preliminary study, and under normal conditions, the project can be defined within 1 month.
Formulation and production phase: depending on complexity, it can take approximately 3 months.
Commissioning: includes all the startup activities except for the final concession of electrical power (allowing all equipment to operate simultaneously), which marks the final date of the process.
Roberto Español, Technical Director of Life Sciences at Trescal:
Once the project is underway, Trescal comes into play. The duration varies depending on the company’s size and how standardized the process is, but it usually takes between 1 and 2 months to plan how the qualifications will be approached. What prolongs the timeline the most is operator training, as once the certifying company is no longer involved, it’s the operators who control the process’s quality. This training can last between 2 months and 2 years, depending on the prior experience of the staff.
Raul Fernández, Project Engineer at Labsom:
From the project’s start to execution, it can take from 6 to 18 months, depending on the project’s scope and having the project well-defined in advance.
Juan Antonio Márquez, Director of Technical Office at Agropharm Projects:
It’s worth noting that most of the tasks overlap. In general, the entire project typically lasts around 2 years.
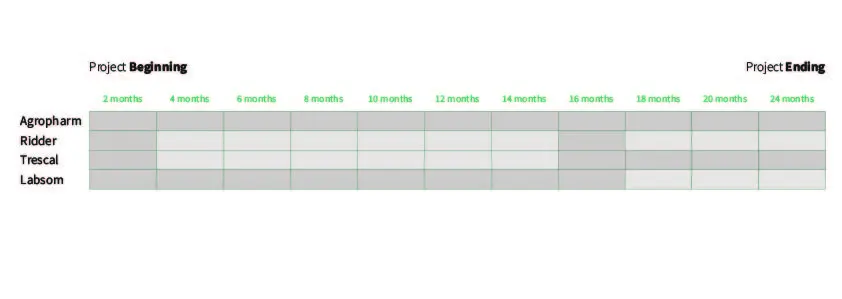
Figure 3. Timeline of Companies for Project Development.
Acknowledgements:
- Eugenio Pérez de Lema, Director of Farmaforum.
- Marcos Muiños Docampo, Coordinator and Manager of Farmaforum.
- Marta Rodríguez Vélez, Conference Coordinator of Farmaforum.
