Jaime Gil Robles, business promotion and development advisor, moderator of this round table.
Agropharm defines itself as a company that integrates turnkey projects for medical cannabis production facilities, that is, from the moment the investor appears with the intention of launching a business until the facility is fully operational.
Agropharm places the investor in an industrial-pharmaceutical environment, so the company’s role is to help these people become familiar with all the processes involved, as well as the commercial volume that will be generated by this agro-industrial activity.
Mar Díaz, Technical Validation Manager at TRESCAL:
From no point of view should we forget that a greenhouse or medical cannabis facility is a medicine factory. Frankenstein projects, those that lack a coordinating entity, do not meet the necessary regulatory expectations, such as the regulations that guarantee good agricultural and pharmaceutical practices such as GACP and GMP. The pharmaceutical sector for medicinal use must be treated with all its nuances in mind, including the medicinal cannabis sector, which should be treated as another medicine.
The QMS must have a proper documentary structure, with a quality manual, and SOP’s oriented to comply with the guidelines of GACP, GMP, GDP.
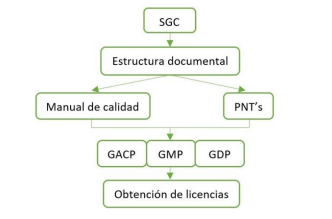
Figure 1. Compliance requirements
We must identify and know the critical points of each stage of the process, from cultivation, harvesting, cutting, to processing, conditioning, storage and distribution, in order to provide the process with the appropriate facilities, as well as the most suitable control strategy. It is an important point to review here the degree/level of automation of the systems/equipment for the control of their operation, and continuous improvement of the processes.
Therefore, it is important to apply “critical thinking” and “Quality Risk Management (QRM)”.
On the other hand, we must know the use that the holder of the manufacturing authorisation is going to give to the cannabis for medicinal use (active substance, intermediate product or finished product) in order to know the level of regulatory requirement GMP Part I (intermediate product, finished product) or GMP Part II (active substance). See ANNEX 7 of the EU GMP.
We must have a Technical Report of the installation and a VMP (Validation Master Plan) with the description of the unit to be validated with details of its systems and equipment.
In this type of industry it is very important to consider all the previous work before starting up the installation in order to obtain the corresponding manufacturing authorisation in accordance with the current regulatory framework. Trescal validates the systems and processes, qualifies the equipment, as well as develops the required documentation related to the Quality Management System (GMP, GDP, GACP), Bezanilla & Renedo lawyers corroborate local, regional and national standards and Agropharm prepares the technical documentation and design of the facilities.
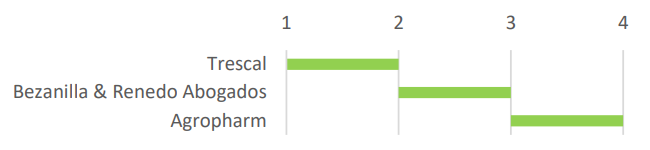
Figure 2. Chronology of companies for the development of a project
From our side, Agropharm has the experience of executing different processing plants in Spain and Portugal.
Rafael Rey, Agropharm Projects’ CEO:
Our objective as a company is to integrate all the participants in carrying out construction projects, Agropharm has a constant presence in the execution of the work, which transmits to the client greater transparency and peace of mind, and makes it possible to receive everything necessary for the evaluation of the work of execution management.
By managing to integrate all the companies, a single channel of communication is defined so that the objectives of the client’s investment strategy are met.
Contrary to the Frankenstein philosophy, we try to avoid that, at the initiative of the investor, his own means are used to execute the project, thus generating uncertainties and, ultimately, insecurities among all the participants of the project.
Antonio Bezanilla, lawyer and counsel at Bezanilla&Renedo Abogados:
In cannabis sector projects, it is a common mistake to start execution phases with a lack of coordination or programming of the work, especially in terms of the study of the functionality and viability of the facilities, which is usually the first cause of problems with the authorisations and validations that are essential to successfully obtain the production licence.
In most projects of this type, it is likely that clashes will arise between the different parties involved in the project, which is why the integration and backing of a proven model such as Agropharm’s means that the client has the certainty and peace of mind of working safely, in exchange for which the integrating company indirectly manages the frustration generated in the client. The coordination of a work or a project and its materialisation is synonymous with happiness.
José Maraver, founder and partner of GreenDonation Kannabeira:
In our case, we came to Agropharm with a complete project with a large part of the work decided, we were looking for a company that could integrate all the services that a project of this level demands, and with Agropharm we have avoided problems and difficulties that we would have had if we had not left all the coordination work in their hands.
From the client’s point of view, it is much easier for the development of the project to deal with one company coordinating the project rather than ten companies, each of which has a different method of working.
Roberto Español, LifeSciences Technical Director at Trescal:
In this type of project, for these facilities, one of the most important aspects is to have an adequate Quality Management System in accordance with the GMP/GDP/GACP framework, as well as to have adequate documentation relating to our facilities and equipment. It is common to find projects where this is not taken into account and seems to be more tedious as it is work oriented to the collection and obtaining of documentation of the processes and installations.
We must not forget that the objective or purpose of these installations is to obtain medicines that require measures that guarantee reliability and that is developed on a continuous improvement basis, by means of controls that aim to obtain results to improve operation and, thus, avoid known and unknown risks that may appear.
A quality system in accordance with GMP, GDP and GACP, becomes a requirement to keep our production plant open, so it is essential that this system is documented, because as they say in the pharmaceutical world “what is not written, does not exist”.
Therefore, the coordination of the project will entail fighting for the resources to ensure that the quality system works.
What problems does a Frankenstein project create?
Rafael Rey, Agropharm Projects‘ CEO:
With the necessary accreditation and the relationship from the outset, there will probably be problems of understanding. Our cover letter is like a curriculum vitae, we have several participations in different projects or licences, and we have managed to adapt our way of working to each of them, dealing with the particularities.
At Agropharm, we have the security of working with a base of permanent collaborators, from pre-sales to the end of the execution. For the execution of the project and the tasks, a correct programming and coordination of the different work teams is essential so that, in the end, there is a relationship between the adequate execution time and the cost of the project.
It is in this aspect where a large part of the project funds are at stake, i.e. who is going to accompany me in the execution of the project, who is going to be my partner in the development of all the work, what is the investor or promoter of the installation going to need in order to really have control?
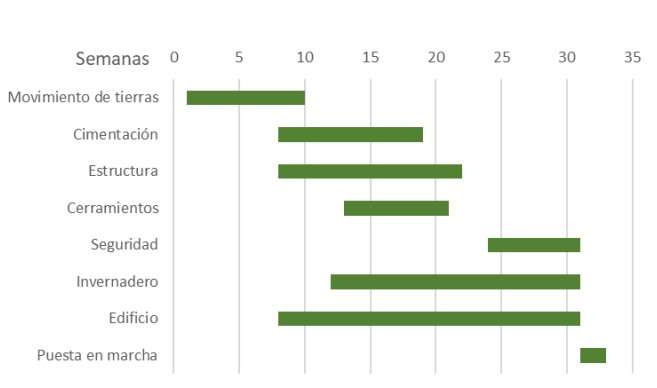
Figure 3. On-site planning
Antonio Bezanilla, lawyer and counsel at Bezanilla&Renedo Abogados:
To bring forward a cannabis project is to generate a pharmaceutical quality system that hates improvisation and lack of coordination and, if there is one thing that is clear, it is that in 9.9 out of 10 Frankenstein projects there is a lack of coordination.
Lawyers, in order to generate a cannabis project, implement the technical report and take it to legal recourse, with pre-contractual preparation work, which takes thousands of hours to get everything right.
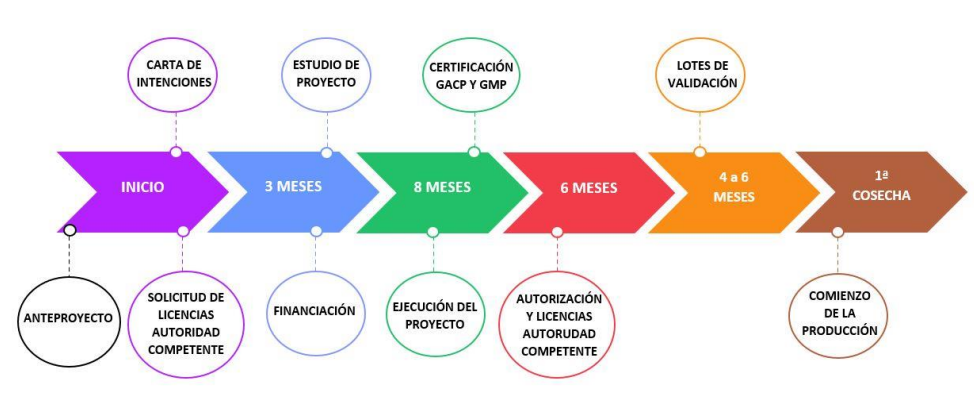
Figure 4. Project planning
We are going to work with schedule 1 drugs, which creates a problem for us in terms of project financing, as they are subject to the money laundering law, and another problem is that our road map fits in with the financial plan.
The lack of coordination is a reason for a drop in the production of pharmaceutical assets, and this starts before the financing if the efficient use of resources is not declared. Our recommendation is to avoid, as far as possible, the lack of coordination through a proven model.
José Maraver, founder and partner of GreenDonation Kannabeira:
Some of the problems that we may encounter when carrying out a project are not considering a fire plan or the electrical supply, problems during the work, even after 2 or 3 years of execution if it is not well designed, or not having anyone with whom to share the project from the legal base, such as pharmaceutical validation issues.
Ideally, you want to create a strong team and coalition, and outsource the work to companies you can trust, i.e. that you can see their work and they understand what you want, both today and in the years to come as the product evolves.
Jaime Gil Robles, promotion and business development consultant:
For the case of Kannabeira, it took 18 months to find the place where to carry out the project, because the site must meet a series of legal requirements and conditions, i.e. you have to talk to the mayor and the autonomous community to get them to give the permits, check the electricity supply, water supply or the type of soil, among others.
Therefore, determination and previous work prevent possible situations that could prevent the project from being carried out.
How to avoid a Frankenstein?
Rafael Rey, Agropharm Projects‘ CEO:
There are very valid companies in the sector that select the technician who will be on site supervising the project and, at the moment of truth, when the work is practically done, they realise that, for example, the electricity needed by the industry is not arriving.
Agropharm, as a construction company, can provide the necessary foresight and viability for the construction, since, through our experience, we guarantee the validation of the chain of events that make the execution of the project possible.
Roberto Español, LifeSciences Technical Director at Trescal:
Frankenstein projects are avoided with experience, to know the projects and the world in which you are moving, and the provision of a person responsible for the validation of the project and its coordination in order to be able to demand the appropriate documentation and compliance from suppliers. This last figure is called Project Manager. We must therefore have a team of specialised technicians who are responsible for coordinating and demanding specific requirements from each of the suppliers. It is the same as an optional management, but with a more predictive approach and focused on regulatory compliance.
The problem that a Frankenstein project generates for us is the difficulty in establishing our QMS in accordance with the GACP/GMP/GDP, as well as in carrying out the validation and qualification files of our systems/equipment due to the lack of adequate documentation provided by the suppliers, as they are non-expert suppliers with a lack of training and experience in the sector/matter.
As a result, we are faced with problems such as the existence of critical deviations during the validation process, not achieving the conformity of the installation, delays in its conformity, or that all of the above have repercussions on higher costs.
Keep in mind that this is a controversial issue and inspectors will look at it with a double lens due to lack of experience. The approach has to be holistic, i.e. know what each supplier is going to give you and how to enforce it and demonstrate that what they are doing is in line with what the pharmaceutical authorities are going to demand from you afterwards.
Antonio Bezanilla, lawyer and counsel at Bezanilla&Renedo Abogados:
And if something happens, who are you going to call?
When you don’t know who to call, it seems that calling a lawyer is never a bad idea, you can always have a chance to fix it. When we suffered the first Frankenstein project, we realised that there were always failures in the greenhouse and it was always someone else’s fault.
So, to avoid Frankenstein projects, the first thing is to follow a proven model, it’s not witchcraft, it’s regulatory technique both legal and quality, and the second thing is to work with a single management team to know whose fault it is and who is in charge of fixing the situation. The winner here is the one who doesn’t go mad.
José Maraver, founder and partner of GreenDonation Kannabeira:
What I would recommend to a project management is to dedicate all the time necessary to know and design, and find a partner to carry it out. Not on the business side, but on the implementation side. There is an infinite list of aspects to take into account in the project, such as who is going to be your builder, who is going to move your clean rooms, where are you going to get the genetics from, who is your client, who is your transformer or who is your distributor.
Cannabis is a plant that can be used industrially to generate thousands of products, so you have to dedicate time to what you know how to do and focus on the final product, because if you don’t, it can cause problems for the product and the market. Let everyone do what they do.
Those who will be in charge of running the industry should first focus on the development of a Business Plan, the application for licences, and the workflow to get the product obtained into a final pharmaceutical form according to its classification and categorisation.
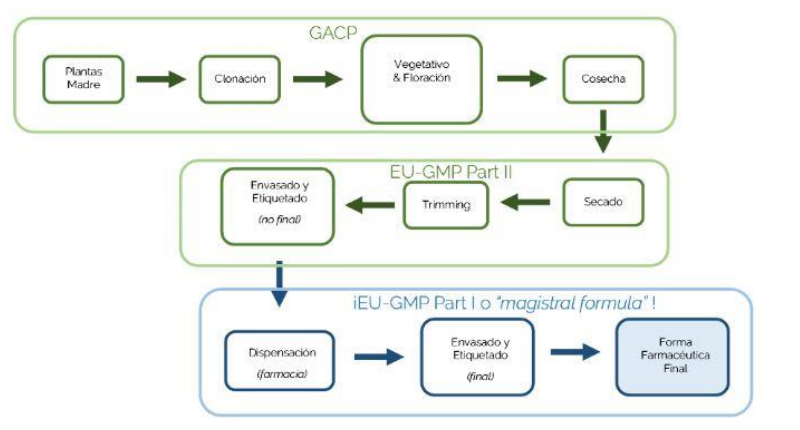
Figure 5. Workflow in the industry
Acknowledgements:
– Eugenio Pérez de Lema, director of Farmaforum.
– Marcos Muiños Docampo, Farmaforum coordinator and manager.
– Marta Rodríguez Vélez, Farmaforum conference coordinator.
